Conserved Binding Mode Database (CBM)
ftp
Proteins evolved through the shuffling of functional domains, and therefore, the same domain can be found in different proteins and species. Interactions between such conserved domains often involve specific, well-determined binding surfaces reflecting their important biological role in a cell. To find biologically relevant interactions we developed a method of systematically comparing and classifying protein domain interactions from the structural data. As a result, a set of conserved binding modes (CBMs) was created using the atomic detail of structure alignment data and the protein domain classification of the Conserved Domain Database. A conserved binding mode is inferred when different members of interacting domain families dock in the same way, such that their structural complexes superimpose well. Such domain interactions with recurring structural themes have greater significance to be biologically relevant, unlike spurious crystal packing interactions.The CBM interaction data provides a list of interacting chains/domains categorized by CDD domain family type and by mode of interaction
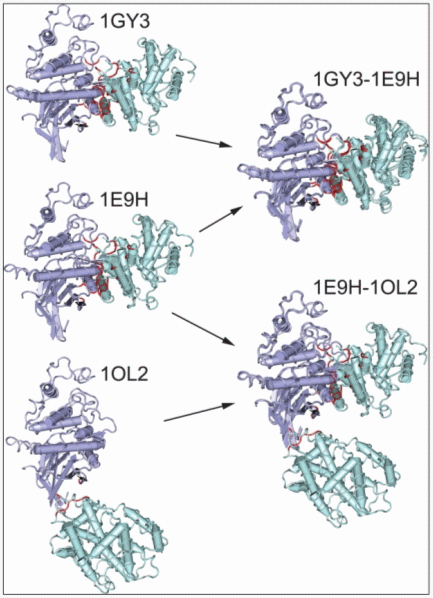 |
The cyclin domain (cd00043) is shown in green interacting with a protein kinase domain (cd00180) in purple to illustrate the definition of CBMs. On the left, three different structures are shown containing this interaction, with interacting residues highlighted in red. On the right, structural superpositions are shown between these structures to determine conserved binding modes. In the alignment of 1GY3 to 1E9H, a sufficient fraction of the interfacial residues overlap, and the two structures create a conserved binding mode. The alignment of 1E9H to 1OL2, however, fails the definition of overlap to be grouped in the same binding mode. The 1OL2 interaction is, in fact, due to crystal packing and is isolated as a nonconserved mode. |
Reference: Shoemaker BA, Panchenko AR, Bryant SH (2006) Finding biologically relevant protein domain interactions: conserved binding mode analysis. Protein Sci 15:352-61